The Periodic Table of Elements
- alkali metal
- alkaline earth metal
- transition metal
- post transition metal
- metalloid
- polyatomic nonmetal
- diatomic nonmetal
- noble gas
- lanthanide
- actinide
H
Hydrogen
1
He
Helium
2
Li
Lithium
3
Be
Beryllium
4
B
Boron
5
C
Carbon
6
N
Nitrogen
7
O
Oxygen
8
F
Fluorine
9
Ne
Neon
10
Na
Sodium
11
Mg
Magnesium
12
Al
Aluminium
13
Si
Silicon
14
P
Phosphorus
15
S
Sulfur
16
Cl
Chlorine
17
Ar
Argon
18
K
Potassium
19
Ca
Calcium
20
Sc
Scandium
21
Ti
Titanium
22
V
Vanadium
23
Cr
Chromium
24
Mn
Manganese
25
Fe
Iron
26
Co
Cobalt
27
Ni
Nickel
28
Cu
Copper
29
Zn
Zinc
30
Ga
Gallium
31
Ge
Germanium
32
As
Arsenic
33
Se
Selenium
34
Br
Bromine
35
Kr
Krypton
36
Rb
Rubidium
37
Sr
Strontium
38
Y
Yttrium
39
Zr
Zirconium
40
Nb
Niobium
41
Mo
Molybdenum
42
Tc
Technetium
43
Ru
Ruthenium
44
Rh
Rhodium
45
Pd
Palladium
46
Ag
Silver
47
Cd
Cadmium
48
In
Indium
49
Sn
Tin
50
Sb
Antimony
51
Te
Tellurium
52
I
Iodine
53
Xe
Xenon
54
Cs
Caesium
55
Ba
Barium
56
Hf
Hafnium
72
Ta
Tantalum
73
W
Tungsten
74
Re
Rhenium
75
Os
Osmium
76
Ir
Iridium
77
Pt
Platinum
78
Au
Gold
79
Hg
Mercury
80
Tl
Thallium
81
Pb
Lead
82
Bi
Bismuth
83
Po
Polonium
84
At
Astatine
85
Rn
Radon
86
Fr
Francium
87
Ra
Radium
88
Rf
Rutherfordium
104
Db
Dubnium
105
Sg
Seaborgium
106
Bh
Bohrium
107
Hs
Hassium
108
Mt
Meitnerium
109
Ds
Darmstadtium
110
Rg
Roentgenium
111
Cn
Copernicium
112
Nh
Nihonium
113
Fl
Flerovium
114
Mc
Moscovium
115
Lv
Livermorium
116
Ts
Tennessine
117
Og
Oganesson
118
La
Lanthanum
57
Ce
Cerium
58
Pr
Praseodymium
59
Nd
Neodymium
60
Pm
Promethium
61
Sm
Samarium
62
Eu
Europium
63
Gd
Gadolinium
64
Tb
Terbium
65
Dy
Dysprosium
66
Ho
Holmium
67
Er
Erbium
68
Tm
Thulium
69
Yb
Ytterbium
70
Lu
Lutetium
71
Ac
Actinium
89
Th
Thorium
90
Pa
Protactinium
91
U
Uranium
92
Np
Neptunium
93
Pu
Plutonium
94
Am
Americium
95
Cm
Curium
96
Bk
Berkelium
97
Cf
Californium
98
Es
Einsteinium
99
Fm
Fermium
100
Md
Mendelevium
101
No
Nobelium
102
Lr
Lawrencium
103
×
Hydrogen
- Category
- diatomic nonmetal
- Appearance
- colorless gas
- Summary
- With an atomic weight of 1.00794 u, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
- Discovered by
- Henry Cavendish
- Melting point
- 13.99 K
- Boiling point
- 20.271 K
- Density
- 0.08988 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Helium
- Category
- noble gas
- Appearance
- colorless gas, exhibiting a red-orange glow when placed in a high-voltage electric field
- Summary
- It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table. Its boiling and melting points are the lowest among all the elements.
- Discovered by
- Pierre Janssen
- Melting point
- 0.95 K
- Boiling point
- 4.222 K
- Density
- 0.1786 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Lithium
- Category
- alkali metal
- Appearance
- silvery-white
- Summary
- It is a soft, silver-white metal belonging to the alkali metal group of chemical elements. Under standard conditions it is the lightest metal and the least dense solid element.
- Discovered by
- Johan August Arfwedson
- Melting point
- 453.65 K
- Boiling point
- 1603 K
- Density
- 0.534 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Beryllium
- Category
- alkaline earth metal
- Appearance
- white-gray metallic
- Summary
- It is created through stellar nucleosynthesis and is a relatively rare element in the universe. It is a divalent element which occurs naturally only in combination with other elements in minerals.
- Discovered by
- Louis Nicolas Vauquelin
- Melting point
- 1560 K
- Boiling point
- 2742 K
- Density
- 1.85 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Boron
- Category
- metalloid
- Appearance
- black-brown
- Summary
- Produced entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, it is a low-abundance element in both the Solar system and the Earth's crust. Boron is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals.
- Discovered by
- Joseph Louis Gay-Lussac
- Melting point
- 2349 K
- Boiling point
- 4200 K
- Density
- 2.08 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Carbon
- Category
- polyatomic nonmetal
- Appearance
- None
- Summary
- On the periodic table, it is the first (row 2) of six elements in column (group) 14, which have in common the composition of their outer electron shell. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.
- Discovered by
- Ancient Egypt
- Melting point
- None K
- Boiling point
- None K
- Density
- 1.821 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Nitrogen
- Category
- diatomic nonmetal
- Appearance
- colorless gas, liquid or solid
- Summary
- It is the lightest pnictogen and at room temperature, it is a transparent, odorless diatomic gas. Nitrogen is a common element in the universe, estimated at about seventh in total abundance in the Milky Way and the Solar System.
- Discovered by
- Daniel Rutherford
- Melting point
- 63.15 K
- Boiling point
- 77.355 K
- Density
- 1.251 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Oxygen
- Category
- diatomic nonmetal
- Appearance
- None
- Summary
- It is a member of the chalcogen group on the periodic table and is a highly reactive nonmetal and oxidizing agent that readily forms compounds (notably oxides) with most elements. By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium.
- Discovered by
- Carl Wilhelm Scheele
- Melting point
- 54.36 K
- Boiling point
- 90.188 K
- Density
- 1.429 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Fluorine
- Category
- diatomic nonmetal
- Appearance
- None
- Summary
- It is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. As the most electronegative element, it is extremely reactive:almost all other elements, including some noble gases, form compounds with fluorine.
- Discovered by
- André-Marie Ampère
- Melting point
- 53.48 K
- Boiling point
- 85.03 K
- Density
- 1.696 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Neon
- Category
- noble gas
- Appearance
- colorless gas exhibiting an orange-red glow when placed in a high voltage electric field
- Summary
- It is in group 18 (noble gases) of the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air.
- Discovered by
- Morris Travers
- Melting point
- 24.56 K
- Boiling point
- 27.104 K
- Density
- 0.9002 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
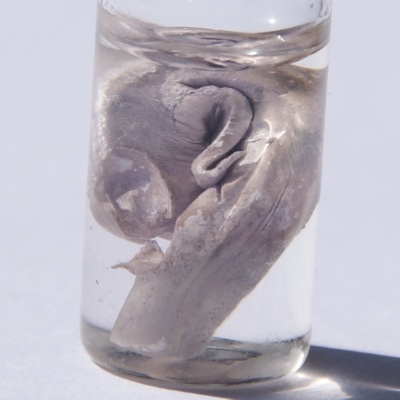
Sodium
- Category
- alkali metal
- Appearance
- silvery white metallic
- Summary
- It is a soft, silver-white, highly reactive metal. In the Periodic table it is in column 1 (alkali metals), and shares with the other six elements in that column that it has a single electron in its outer shell, which it readily donates, creating a positively charged atom - a cation.
- Discovered by
- Humphry Davy
- Melting point
- 370.944 K
- Boiling point
- 1156.09 K
- Density
- 0.968 g/cm3
- Read more at:
- Wikipedia
- Image
- More images and info:
- here
×
Magnesium
- Category
- alkaline earth metal
- Appearance
- shiny grey solid
- Summary
- It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (Group 2, or alkaline earth metals) of the periodic table:they each have the same electron configuration in their outer electron shell producing a similar crystal structure. Magnesium is the ninth most abundant element in the universe.
- Discovered by
- Joseph Black
- Melting point
- 923 K
- Boiling point
- 1363 K
- Density
- 1.738 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Aluminium
- Category
- post-transition metal
- Appearance
- silvery gray metallic
- Summary
- It is a silvery-white, soft, nonmagnetic, ductile metal. Aluminium is the third most abundant element (after oxygen and silicon), and the most abundant metal, in the Earth's crust.
- Discovered by
- None
- Melting point
- 933.47 K
- Boiling point
- 2743 K
- Density
- 2.7 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Silicon
- Category
- metalloid
- Appearance
- crystalline, reflective with bluish-tinged faces
- Summary
- It is a tetravalent metalloid, more reactive than germanium, the metalloid directly below it in the table. Controversy about silicon's character dates to its discovery.
- Discovered by
- Jöns Jacob Berzelius
- Melting point
- 1687 K
- Boiling point
- 3538 K
- Density
- 2.329 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Phosphorus
- Category
- polyatomic nonmetal
- Appearance
- colourless, waxy white, yellow, scarlet, red, violet, black
- Summary
- As an element, phosphorus exists in two major forms—white phosphorus and red phosphorus—but due to its high reactivity, phosphorus is never found as a free element on Earth. Instead phosphorus-containing minerals are almost always present in their maximally oxidised state, as inorganic phosphate rocks.
- Discovered by
- Hennig Brand
- Melting point
- None K
- Boiling point
- None K
- Density
- None g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Sulfur
- Category
- polyatomic nonmetal
- Appearance
- lemon yellow sintered microcrystals
- Summary
- It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8.
- Discovered by
- Ancient china
- Melting point
- 388.36 K
- Boiling point
- 717.8 K
- Density
- 2.07 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Chlorine
- Category
- diatomic nonmetal
- Appearance
- pale yellow-green gas
- Summary
- It also has a relative atomic mass of 35.5. Chlorine is in the halogen group (17) and is the second lightest halogen following fluorine.
- Discovered by
- Carl Wilhelm Scheele
- Melting point
- 171.6 K
- Boiling point
- 239.11 K
- Density
- 3.2 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Argon
- Category
- noble gas
- Appearance
- colorless gas exhibiting a lilac/violet glow when placed in a high voltage electric field
- Summary
- It is in group 18 of the periodic table and is a noble gas. Argon is the third most common gas in the Earth's atmosphere, at 0.934% (9,340 ppmv), making it over twice as abundant as the next most common atmospheric gas, water vapor (which averages about 4000 ppmv, but varies greatly), and 23 times as abundant as the next most common non-condensing atmospheric gas, carbon dioxide (400 ppmv), and more than 500 times as abundant as the next most common noble gas, neon (18 ppmv).
- Discovered by
- Lord Rayleigh
- Melting point
- 83.81 K
- Boiling point
- 87.302 K
- Density
- 1.784 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Potassium
- Category
- alkali metal
- Appearance
- silvery gray
- Summary
- It was first isolated from potash, the ashes of plants, from which its name is derived. In the Periodic table, potassium is one of seven elements in column (group) 1 (alkali metals):they all have a single valence electron in their outer electron shell, which they readily give up to create an atom with a positive charge - a cation, and combine with anions to form salts.
- Discovered by
- Humphry Davy
- Melting point
- 336.7 K
- Boiling point
- 1032 K
- Density
- 0.862 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Calcium
- Category
- alkaline earth metal
- Appearance
- None
- Summary
- Calcium is a soft gray alkaline earth metal, fifth-most-abundant element by mass in the Earth's crust. The ion Ca2+ is also the fifth-most-abundant dissolved ion in seawater by both molarity and mass, after sodium, chloride, magnesium, and sulfate.
- Discovered by
- Humphry Davy
- Melting point
- 1115 K
- Boiling point
- 1757 K
- Density
- 1.55 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Scandium
- Category
- transition metal
- Appearance
- silvery white
- Summary
- A silvery-white metallic d-block element, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia.
- Discovered by
- Lars Fredrik Nilson
- Melting point
- 1814 K
- Boiling point
- 3109 K
- Density
- 2.985 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Titanium
- Category
- transition metal
- Appearance
- silvery grey-white metallic
- Summary
- It is a lustrous transition metal with a silver color, low density and high strength. It is highly resistant to corrosion in sea water, aqua regia and chlorine.
- Discovered by
- William Gregor
- Melting point
- 1941 K
- Boiling point
- 3560 K
- Density
- 4.506 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Vanadium
- Category
- transition metal
- Appearance
- blue-silver-grey metal
- Summary
- It is a hard, silvery grey, ductile and malleable transition metal. The element is found only in chemically combined form in nature, but once isolated artificially, the formation of an oxide layer stabilizes the free metal somewhat against further oxidation.
- Discovered by
- Andrés Manuel del Río
- Melting point
- 2183 K
- Boiling point
- 3680 K
- Density
- 6.0 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Chromium
- Category
- transition metal
- Appearance
- silvery metallic
- Summary
- It is the first element in Group 6. It is a steely-gray, lustrous, hard and brittle metal which takes a high polish, resists tarnishing, and has a high melting point.
- Discovered by
- Louis Nicolas Vauquelin
- Melting point
- 2180 K
- Boiling point
- 2944 K
- Density
- 7.19 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Manganese
- Category
- transition metal
- Appearance
- silvery metallic
- Summary
- It is not found as a free element in nature; it is often found in combination with iron, and in many minerals. Manganese is a metal with important industrial metal alloy uses, particularly in stainless steels.
- Discovered by
- Torbern Olof Bergman
- Melting point
- 1519 K
- Boiling point
- 2334 K
- Density
- 7.21 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Iron
- Category
- transition metal
- Appearance
- lustrous metallic with a grayish tinge
- Summary
- It is a metal in the first transition series. It is by mass the most common element on Earth, forming much of Earth's outer and inner core.
- Discovered by
- 5000 BC
- Melting point
- 1811 K
- Boiling point
- 3134 K
- Density
- 7.874 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Cobalt
- Category
- transition metal
- Appearance
- hard lustrous gray metal
- Summary
- Like nickel, cobalt in the Earth's crust is found only in chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.
- Discovered by
- Georg Brandt
- Melting point
- 1768 K
- Boiling point
- 3200 K
- Density
- 8.9 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Nickel
- Category
- transition metal
- Appearance
- lustrous, metallic, and silver with a gold tinge
- Summary
- It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile.
- Discovered by
- Axel Fredrik Cronstedt
- Melting point
- 1728 K
- Boiling point
- 3003 K
- Density
- 8.908 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Copper
- Category
- transition metal
- Appearance
- red-orange metallic luster
- Summary
- It is a soft, malleable and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a reddish-orange color.
- Discovered by
- Middle East
- Melting point
- 1357.77 K
- Boiling point
- 2835 K
- Density
- 8.96 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Zinc
- Category
- transition metal
- Appearance
- silver-gray
- Summary
- It is the first element of group 12 of the periodic table. In some respects zinc is chemically similar to magnesium:its ion is of similar size and its only common oxidation state is +2.
- Discovered by
- India
- Melting point
- 692.68 K
- Boiling point
- 1180 K
- Density
- 7.14 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Gallium
- Category
- post-transition metal
- Appearance
- silver-white
- Summary
- Elemental gallium does not occur in free form in nature, but as the gallium(III) compounds that are in trace amounts in zinc ores and in bauxite. Gallium is a soft, silvery metal, and elemental gallium is a brittle solid at low temperatures, and melts at 29.76 °C (85.57 °F) (slightly above room temperature).
- Discovered by
- Lecoq de Boisbaudran
- Melting point
- 302.9146 K
- Boiling point
- 2673 K
- Density
- 5.91 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Germanium
- Category
- metalloid
- Appearance
- grayish-white
- Summary
- It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. Purified germanium is a semiconductor, with an appearance most similar to elemental silicon.
- Discovered by
- Clemens Winkler
- Melting point
- 1211.4 K
- Boiling point
- 3106 K
- Density
- 5.323 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Arsenic
- Category
- metalloid
- Appearance
- metallic grey
- Summary
- Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. Arsenic is a metalloid.
- Discovered by
- Bronze Age
- Melting point
- None K
- Boiling point
- None K
- Density
- 5.727 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Selenium
- Category
- polyatomic nonmetal
- Appearance
- black, red, and gray (not pictured) allotropes
- Summary
- It is a nonmetal with properties that are intermediate between those of its periodic table column-adjacent chalcogen elements sulfur and tellurium. It rarely occurs in its elemental state in nature, or as pure ore compounds.
- Discovered by
- Jöns Jakob Berzelius
- Melting point
- 494 K
- Boiling point
- 958 K
- Density
- 4.81 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Bromine
- Category
- diatomic nonmetal
- Appearance
- None
- Summary
- It is a halogen. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826.
- Discovered by
- Antoine Jérôme Balard
- Melting point
- 265.8 K
- Boiling point
- 332.0 K
- Density
- 23.1028 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Krypton
- Category
- noble gas
- Appearance
- colorless gas, exhibiting a whitish glow in a high electric field
- Summary
- It is a member of group 18 (noble gases) elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquefied air, and is often used with other rare gases in fluorescent lamps.
- Discovered by
- William Ramsay
- Melting point
- 115.78 K
- Boiling point
- 119.93 K
- Density
- 3.749 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Rubidium
- Category
- alkali metal
- Appearance
- grey white
- Summary
- Rubidium is a soft, silvery-white metallic element of the alkali metal group, with an atomic mass of 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other alkali metals, such as very rapid oxidation in air.
- Discovered by
- Robert Bunsen
- Melting point
- 312.45 K
- Boiling point
- 961 K
- Density
- 1.532 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Strontium
- Category
- alkaline earth metal
- Appearance
- None
- Summary
- An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when it is exposed to air.
- Discovered by
- William Cruickshank (chemist)
- Melting point
- 1050 K
- Boiling point
- 1650 K
- Density
- 2.64 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Yttrium
- Category
- transition metal
- Appearance
- silvery white
- Summary
- It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is never found in nature as a free element.
- Discovered by
- Johan Gadolin
- Melting point
- 1799 K
- Boiling point
- 3203 K
- Density
- 4.472 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Zirconium
- Category
- transition metal
- Appearance
- silvery white
- Summary
- The name of zirconium is taken from the name of the mineral zircon, the most important source of zirconium. The word zircon comes from the Persian word zargun زرگون, meaning "gold-colored".
- Discovered by
- Martin Heinrich Klaproth
- Melting point
- 2128 K
- Boiling point
- 4650 K
- Density
- 6.52 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Niobium
- Category
- transition metal
- Appearance
- gray metallic, bluish when oxidized
- Summary
- It is a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite. The name comes from Greek mythology:Niobe, daughter of Tantalus since it is so similar to tantalum.
- Discovered by
- Charles Hatchett
- Melting point
- 2750 K
- Boiling point
- 5017 K
- Density
- 8.57 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Molybdenum
- Category
- transition metal
- Appearance
- gray metallic
- Summary
- The name is from Neo-Latin molybdaenum, from Ancient Greek Μόλυβδος molybdos, meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele.
- Discovered by
- Carl Wilhelm Scheele
- Melting point
- 2896 K
- Boiling point
- 4912 K
- Density
- 10.28 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Technetium
- Category
- transition metal
- Appearance
- shiny gray metal
- Summary
- It is the element with the lowest atomic number in the periodic table that has no stable isotopes:every form of it is radioactive. Nearly all technetium is produced synthetically, and only minute amounts are found in nature.
- Discovered by
- Emilio Segrè
- Melting point
- 2430 K
- Boiling point
- 4538 K
- Density
- 11 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Ruthenium
- Category
- transition metal
- Appearance
- silvery white metallic
- Summary
- It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemicals.
- Discovered by
- Karl Ernst Claus
- Melting point
- 2607 K
- Boiling point
- 4423 K
- Density
- 12.45 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Rhodium
- Category
- transition metal
- Appearance
- silvery white metallic
- Summary
- It is a rare, silvery-white, hard, and chemically inert transition metal. It is a member of the platinum group.
- Discovered by
- William Hyde Wollaston
- Melting point
- 2237 K
- Boiling point
- 3968 K
- Density
- 12.41 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Palladium
- Category
- transition metal
- Appearance
- silvery white
- Summary
- It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas.
- Discovered by
- William Hyde Wollaston
- Melting point
- 1828.05 K
- Boiling point
- 3236 K
- Density
- 12.023 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Silver
- Category
- transition metal
- Appearance
- lustrous white metal
- Summary
- A soft, white, lustrous transition metal, it possesses the highest electrical conductivity, thermal conductivity and reflectivity of any metal. The metal occurs naturally in its pure, free form (native silver), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite.
- Discovered by
- unknown, before 5000 BC
- Melting point
- 1234.93 K
- Boiling point
- 2435 K
- Density
- 10.49 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Cadmium
- Category
- transition metal
- Appearance
- silvery bluish-gray metallic
- Summary
- This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it prefers oxidation state +2 in most of its compounds and like mercury it shows a low melting point compared to transition metals.
- Discovered by
- Karl Samuel Leberecht Hermann
- Melting point
- 594.22 K
- Boiling point
- 1040 K
- Density
- 8.65 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Indium
- Category
- post-transition metal
- Appearance
- silvery lustrous gray
- Summary
- It is a post-transition metallic element that is rare in Earth's crust. The metal is very soft, malleable and easily fusible, with a melting point higher than sodium, but lower than lithium or tin.
- Discovered by
- Ferdinand Reich
- Melting point
- 429.7485 K
- Boiling point
- 2345 K
- Density
- 7.31 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Tin
- Category
- post-transition metal
- Appearance
- silvery-white (beta, β) or gray (alpha, α)
- Summary
- It is a main group metal in group 14 of the periodic table. Tin shows a chemical similarity to both neighboring group-14 elements, germanium and lead, and has two possible oxidation states, +2 and the slightly more stable +4.
- Discovered by
- unknown, before 3500 BC
- Melting point
- 505.08 K
- Boiling point
- 2875 K
- Density
- 7.365 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Antimony
- Category
- metalloid
- Appearance
- silvery lustrous gray
- Summary
- A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were used for cosmetics; metallic antimony was also known, but it was erroneously identified as lead upon its discovery.
- Discovered by
- unknown, before 3000 BC
- Melting point
- 903.78 K
- Boiling point
- 1908 K
- Density
- 6.697 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Tellurium
- Category
- metalloid
- Appearance
- None
- Summary
- It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur.
- Discovered by
- Franz-Joseph Müller von Reichenstein
- Melting point
- 722.66 K
- Boiling point
- 1261 K
- Density
- 6.24 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Iodine
- Category
- diatomic nonmetal
- Appearance
- lustrous metallic gray, violet as a gas
- Summary
- The name is from Greek ἰοειδής ioeidēs, meaning violet or purple, due to the color of iodine vapor. Iodine and its compounds are primarily used in nutrition, and industrially in the production of acetic acid and certain polymers.
- Discovered by
- Bernard Courtois
- Melting point
- 386.85 K
- Boiling point
- 457.4 K
- Density
- 4.933 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Xenon
- Category
- noble gas
- Appearance
- colorless gas, exhibiting a blue glow when placed in a high voltage electric field
- Summary
- It is a colorless, dense, odorless noble gas, that occurs in the Earth's atmosphere in trace amounts. Although generally unreactive, xenon can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized.
- Discovered by
- William Ramsay
- Melting point
- 161.4 K
- Boiling point
- 165.051 K
- Density
- 5.894 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Caesium
- Category
- alkali metal
- Appearance
- silvery gold
- Summary
- It is a soft, silvery-gold alkali metal with a melting point of 28 °C (82 °F), which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium is an alkali metal and has physical and chemical properties similar to those of rubidium and potassium.
- Discovered by
- Robert Bunsen
- Melting point
- 301.7 K
- Boiling point
- 944 K
- Density
- 1.93 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Barium
- Category
- alkaline earth metal
- Appearance
- None
- Summary
- It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Because of its high chemical reactivity barium is never found in nature as a free element.
- Discovered by
- Carl Wilhelm Scheele
- Melting point
- 1000 K
- Boiling point
- 2118 K
- Density
- 3.51 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Hafnium
- Category
- transition metal
- Appearance
- steel gray
- Summary
- A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1923, making it the penultimate stable element to be discovered (rhenium was identified two years later).
- Discovered by
- Dirk Coster
- Melting point
- 2506 K
- Boiling point
- 4876 K
- Density
- 13.31 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Tantalum
- Category
- transition metal
- Appearance
- gray blue
- Summary
- Previously known as tantalium, its name comes from Tantalus, an antihero from Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion-resistant.
- Discovered by
- Anders Gustaf Ekeberg
- Melting point
- 3290 K
- Boiling point
- 5731 K
- Density
- 16.69 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Tungsten
- Category
- transition metal
- Appearance
- grayish white, lustrous
- Summary
- The word tungsten comes from the Swedish language tung sten, which directly translates to heavy stone. Its name in Swedish is volfram, however, in order to distinguish it from scheelite, which in Swedish is alternatively named tungsten.
- Discovered by
- Carl Wilhelm Scheele
- Melting point
- 3695 K
- Boiling point
- 6203 K
- Density
- 19.25 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Rhenium
- Category
- transition metal
- Appearance
- silvery-grayish
- Summary
- It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust.
- Discovered by
- Masataka Ogawa
- Melting point
- 3459 K
- Boiling point
- 5869 K
- Density
- 21.02 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Osmium
- Category
- transition metal
- Appearance
- silvery, blue cast
- Summary
- It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element, with a density of 22.59 g/cm3.
- Discovered by
- Smithson Tennant
- Melting point
- 3306 K
- Boiling point
- 5285 K
- Density
- 22.59 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Iridium
- Category
- transition metal
- Appearance
- silvery white
- Summary
- A very hard, brittle, silvery-white transition metal of the platinum group, iridium is generally credited with being the second densest element (after osmium) based on measured density, although calculations involving the space lattices of the elements show that iridium is denser. It is also the most corrosion-resistant metal, even at temperatures as high as 2000 °C. Although only certain molten salts and halogens are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable.
- Discovered by
- Smithson Tennant
- Melting point
- 2719 K
- Boiling point
- 4403 K
- Density
- 22.56 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Platinum
- Category
- transition metal
- Appearance
- silvery white
- Summary
- It is a dense, malleable, ductile, highly unreactive, precious, gray-white transition metal. Its name is derived from the Spanish term platina, which is literally translated into "little silver".
- Discovered by
- Antonio de Ulloa
- Melting point
- 2041.4 K
- Boiling point
- 4098 K
- Density
- 21.45 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Gold
- Category
- transition metal
- Appearance
- metallic yellow
- Summary
- In its purest form, it is a bright, slightly reddish yellow, dense, soft, malleable and ductile metal. Chemically, gold is a transition metal and a group 11 element.
- Discovered by
- Middle East
- Melting point
- 1337.33 K
- Boiling point
- 3243 K
- Density
- 19.3 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Mercury
- Category
- transition metal
- Appearance
- silvery
- Summary
- It is commonly known as quicksilver and was formerly named hydrargyrum (/haɪˈdrɑːrdʒərəm/). A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
- Discovered by
- unknown, before 2000 BCE
- Melting point
- 234.321 K
- Boiling point
- 629.88 K
- Density
- 13.534 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Thallium
- Category
- post-transition metal
- Appearance
- silvery white
- Summary
- This soft gray post-transition metal is not found free in nature. When isolated, it resembles tin, but discolors when exposed to air.
- Discovered by
- William Crookes
- Melting point
- 577 K
- Boiling point
- 1746 K
- Density
- 11.85 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Lead
- Category
- post-transition metal
- Appearance
- metallic gray
- Summary
- Lead is a soft, malleable and heavy post-transition metal. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed to air.
- Discovered by
- Middle East
- Melting point
- 600.61 K
- Boiling point
- 2022 K
- Density
- 11.34 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
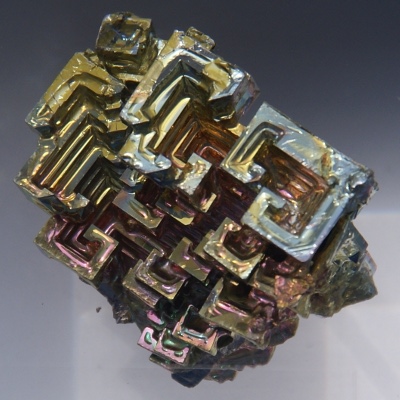
Bismuth
- Category
- post-transition metal
- Appearance
- lustrous silver
- Summary
- Bismuth, a pentavalent post-transition metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally, although its sulfide and oxide form important commercial ores.
- Discovered by
- Claude François Geoffroy
- Melting point
- 544.7 K
- Boiling point
- 1837 K
- Density
- 9.78 g/cm3
- Read more at:
- Wikipedia
- Image
- More images and info:
- here
×
Polonium
- Category
- post-transition metal
- Appearance
- silvery
- Summary
- A rare and highly radioactive element with no stable isotopes, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Applications of polonium are few.
- Discovered by
- Pierre Curie
- Melting point
- 527 K
- Boiling point
- 1235 K
- Density
- 9.196 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Astatine
- Category
- metalloid
- Appearance
- unknown, probably metallic
- Summary
- It occurs on Earth as the decay product of various heavier elements. All its isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours.
- Discovered by
- Dale R. Corson
- Melting point
- 575 K
- Boiling point
- 610 K
- Density
- 26.35 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
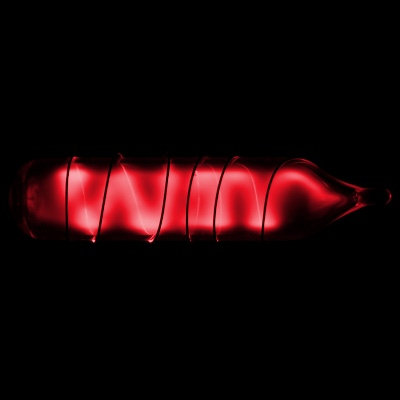
Radon
- Category
- noble gas
- Appearance
- colorless gas, occasionally glows green or red in discharge tubes
- Summary
- It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as a decay product of radium. Its most stable isotope, 222Rn, has a half-life of 3.8 days.
- Discovered by
- Friedrich Ernst Dorn
- Melting point
- 202 K
- Boiling point
- 211.5 K
- Density
- 9.73 g/cm3
- Read more at:
- Wikipedia
- Image
- More images and info:
- here
×
Francium
- Category
- alkali metal
- Appearance
- None
- Summary
- It used to be known as eka-caesium and actinium K. It is the second-least electronegative element, behind only caesium. Francium is a highly radioactive metal that decays into astatine, radium, and radon.
- Discovered by
- Marguerite Perey
- Melting point
- 300 K
- Boiling point
- 950 K
- Density
- 1.87 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Radium
- Category
- alkaline earth metal
- Appearance
- silvery white metallic
- Summary
- It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is almost colorless, but it readily combines with nitrogen (rather than oxygen) on exposure to air, forming a black surface layer of radium nitride (Ra3N2).
- Discovered by
- Pierre Curie
- Melting point
- 1233 K
- Boiling point
- 2010 K
- Density
- 5.5 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Rutherfordium
- Category
- transition metal
- Appearance
- None
- Summary
- It is a synthetic element (an element that can be created in a laboratory but is not found in nature) and radioactive; the most stable known isotope, 267Rf, has a half-life of approximately 1.3 hours. In the periodic table of the elements, it is a d - block element and the second of the fourth - row transition elements.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- 2400 K
- Boiling point
- 5800 K
- Density
- 23.2 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Dubnium
- Category
- transition metal
- Appearance
- None
- Summary
- It is named after the town of Dubna in Russia (north of Moscow), where it was first produced. It is a synthetic element (an element that can be created in a laboratory but is not found in nature) and radioactive; the most stable known isotope, dubnium-268, has a half-life of approximately 28 hours.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- None K
- Boiling point
- None K
- Density
- 29.3 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Seaborgium
- Category
- transition metal
- Appearance
- None
- Summary
- Its most stable isotope 271Sg has a half-life of 1.9 minutes. A more recently discovered isotope 269Sg has a potentially slightly longer half-life (ca.
- Discovered by
- Lawrence Berkeley National Laboratory
- Melting point
- None K
- Boiling point
- None K
- Density
- 35.0 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Bohrium
- Category
- transition metal
- Appearance
- None
- Summary
- It is named after Danish physicist Niels Bohr. It is a synthetic element (an element that can be created in a laboratory but is not found in nature) and radioactive; the most stable known isotope, 270Bh, has a half-life of approximately 61 seconds.
- Discovered by
- Gesellschaft für Schwerionenforschung
- Melting point
- None K
- Boiling point
- None K
- Density
- 37.1 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Hassium
- Category
- transition metal
- Appearance
- None
- Summary
- It is a synthetic element (an element that can be created in a laboratory but is not found in nature) and radioactive; the most stable known isotope, 269Hs, has a half-life of approximately 9.7 seconds, although an unconfirmed metastable state, 277mHs, may have a longer half-life of about 130 seconds. More than 100 atoms of hassium have been synthesized to date.
- Discovered by
- Gesellschaft für Schwerionenforschung
- Melting point
- 126 K
- Boiling point
- None K
- Density
- 40.7 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Meitnerium
- Category
- unknown, probably transition metal
- Appearance
- None
- Summary
- It is an extremely radioactive synthetic element (an element not found in nature that can be created in a laboratory). The most stable known isotope, meitnerium-278, has a half-life of 7.6 seconds.
- Discovered by
- Gesellschaft für Schwerionenforschung
- Melting point
- None K
- Boiling point
- None K
- Density
- 37.4 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Darmstadtium
- Category
- unknown, probably transition metal
- Appearance
- None
- Summary
- It is an extremely radioactive synthetic element. The most stable known isotope, darmstadtium-281, has a half-life of approximately 10 seconds.
- Discovered by
- Gesellschaft für Schwerionenforschung
- Melting point
- None K
- Boiling point
- None K
- Density
- 34.8 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Roentgenium
- Category
- unknown, probably transition metal
- Appearance
- None
- Summary
- It is an extremely radioactive synthetic element (an element that can be created in a laboratory but is not found in nature); the most stable known isotope, roentgenium-282, has a half-life of 2.1 minutes. Roentgenium was first created in 1994 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany.
- Discovered by
- Gesellschaft für Schwerionenforschung
- Melting point
- None K
- Boiling point
- None K
- Density
- 28.7 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Copernicium
- Category
- transition metal
- Appearance
- None
- Summary
- It is an extremely radioactive synthetic element that can only be created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of approximately 29 seconds, but it is possible that this copernicium isotope may have a nuclear isomer with a longer half-life, 8.9 min.
- Discovered by
- Gesellschaft für Schwerionenforschung
- Melting point
- None K
- Boiling point
- 3570 K
- Density
- 23.7 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Nihonium
- Category
- unknown, probably transition metal
- Appearance
- None
- Summary
- It has a symbol Nh. It is a synthetic element (an element that can be created in a laboratory but is not found in nature) and is extremely radioactive; its most stable known isotope, nihonium-286, has a half-life of 20 seconds.
- Discovered by
- RIKEN
- Melting point
- 700 K
- Boiling point
- 1430 K
- Density
- 16 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Flerovium
- Category
- post-transition metal
- Appearance
- None
- Summary
- It is an extremely radioactive synthetic element. The element is named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered in 1998.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- 340 K
- Boiling point
- 420 K
- Density
- 14 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Moscovium
- Category
- unknown, probably post transition metal
- Appearance
- None
- Summary
- It is an extremely radioactive element; its most stable known isotope, moscovium-289, has a half-life of only 220 milliseconds. It is also known as eka-bismuth or simply element 115.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- 670 K
- Boiling point
- 1400 K
- Density
- 13.5 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Livermorium
- Category
- unknown, probably post transition metal
- Appearance
- None
- Summary
- It is an extremely radioactive element that has only been created in the laboratory and has not been observed in nature. The element is named after the Lawrence Livermore National Laboratory in the United States, which collaborated with the Joint Institute for Nuclear Research in Dubna, Russia to discover livermorium in 2000.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- 637780 K
- Boiling point
- 10351135 K
- Density
- 12.9 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Tennessine
- Category
- unknown,probably metalloid
- Appearance
- None
- Summary
- Also known as eka-astatine or element 117, it is the second-heaviest known element and penultimate element of the 7th period of the periodic table. As of 2016, fifteen tennessine atoms have been observed:six when it was first synthesized in 2010, seven in 2012, and two in 2014.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- 623823 K
- Boiling point
- 883 K
- Density
- 7.17 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Oganesson
- Category
- unknown, predicted to be noble gas
- Appearance
- None
- Summary
- It is also known as eka-radon or element 118, and on the periodic table of the elements it is a p-block element and the last one of the 7th period. Oganesson is currently the only synthetic member of group 18.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- None K
- Boiling point
- 35030 K
- Density
- 4.95 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Lanthanum
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It tarnishes rapidly when exposed to air and is soft enough to be cut with a knife. It gave its name to the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table:it is also sometimes considered the first element of the 6th-period transition metals.
- Discovered by
- Carl Gustaf Mosander
- Melting point
- 1193 K
- Boiling point
- 3737 K
- Density
- 6.162 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Cerium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet Ceres (itself named after the Roman goddess of agriculture).
- Discovered by
- Martin Heinrich Klaproth
- Melting point
- 1068 K
- Boiling point
- 3716 K
- Density
- 6.77 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Praseodymium
- Category
- lanthanide
- Appearance
- grayish white
- Summary
- Praseodymium is a soft, silvery, malleable and ductile metal in the lanthanide group. It is valued for its magnetic, electrical, chemical, and optical properties.
- Discovered by
- Carl Auer von Welsbach
- Melting point
- 1208 K
- Boiling point
- 3403 K
- Density
- 6.77 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Neodymium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach.
- Discovered by
- Carl Auer von Welsbach
- Melting point
- 1297 K
- Boiling point
- 3347 K
- Density
- 7.01 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Promethium
- Category
- lanthanide
- Appearance
- metallic
- Summary
- All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements.
- Discovered by
- Chien Shiung Wu
- Melting point
- 1315 K
- Boiling point
- 3273 K
- Density
- 7.26 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Samarium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It is a moderately hard silvery metal that readily oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3.
- Discovered by
- Lecoq de Boisbaudran
- Melting point
- 1345 K
- Boiling point
- 2173 K
- Density
- 7.52 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Europium
- Category
- lanthanide
- Appearance
- None
- Summary
- It was isolated in 1901 and is named after the continent of Europe. It is a moderately hard, silvery metal which readily oxidizes in air and water.
- Discovered by
- Eugène-Anatole Demarçay
- Melting point
- 1099 K
- Boiling point
- 1802 K
- Density
- 5.264 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Gadolinium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It is a silvery-white, malleable and ductile rare-earth metal. It is found in nature only in combined (salt) form.
- Discovered by
- Jean Charles Galissard de Marignac
- Melting point
- 1585 K
- Boiling point
- 3273 K
- Density
- 7.9 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Terbium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It is a silvery-white rare earth metal that is malleable, ductile and soft enough to be cut with a knife. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite.
- Discovered by
- Carl Gustaf Mosander
- Melting point
- 1629 K
- Boiling point
- 3396 K
- Density
- 8.23 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Dysprosium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It is a rare earth element with a metallic silver luster. Dysprosium is never found in nature as a free element, though it is found in various minerals, such as xenotime.
- Discovered by
- Lecoq de Boisbaudran
- Melting point
- 1680 K
- Boiling point
- 2840 K
- Density
- 8.54 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Holmium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- Part of the lanthanide series, holmium is a rare earth element. Holmium was discovered by Swedish chemist Per Theodor Cleve.
- Discovered by
- Marc Delafontaine
- Melting point
- 1734 K
- Boiling point
- 2873 K
- Density
- 8.79 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Erbium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements on Earth. As such, it is a rare earth element which is associated with several other rare elements in the mineral gadolinite from Ytterby in Sweden, where yttrium, ytterbium, and terbium were discovered.
- Discovered by
- Carl Gustaf Mosander
- Melting point
- 1802 K
- Boiling point
- 3141 K
- Density
- 9.066 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Thulium
- Category
- lanthanide
- Appearance
- silvery gray
- Summary
- It is the thirteenth and antepenultimate (third-last) element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other compounds.
- Discovered by
- Per Teodor Cleve
- Melting point
- 1818 K
- Boiling point
- 2223 K
- Density
- 9.32 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Ytterbium
- Category
- lanthanide
- Appearance
- None
- Summary
- It is the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. However, like the other lanthanides, its most common oxidation state is +3, seen in its oxide, halides and other compounds.
- Discovered by
- Jean Charles Galissard de Marignac
- Melting point
- 1097 K
- Boiling point
- 1469 K
- Density
- 6.9 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Lutetium
- Category
- lanthanide
- Appearance
- silvery white
- Summary
- It is a silvery white metal, which resists corrosion in dry, but not in moist air. It is considered the first element of the 6th-period transition metals and the last element in the lanthanide series, and is traditionally counted among the rare earths.
- Discovered by
- Georges Urbain
- Melting point
- 1925 K
- Boiling point
- 3675 K
- Density
- 9.841 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Actinium
- Category
- actinide
- Appearance
- None
- Summary
- It was the first non-primordial radioactive element to be isolated. Polonium, radium and radon were observed before actinium, but they were not isolated until 1902.
- Discovered by
- Friedrich Oskar Giesel
- Melting point
- 1500 K
- Boiling point
- 3500300 K
- Density
- 10 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Thorium
- Category
- actinide
- Appearance
- silvery, often with black tarnish
- Summary
- A radioactive actinide metal, thorium is one of only two significantly radioactive elements that still occur naturally in large quantities as a primordial element (the other being uranium). It was discovered in 1828 by the Norwegian Reverend and amateur mineralogist Morten Thrane Esmark and identified by the Swedish chemist Jöns Jakob Berzelius, who named it after Thor, the Norse god of thunder.
- Discovered by
- Jöns Jakob Berzelius
- Melting point
- 2023 K
- Boiling point
- 5061 K
- Density
- 11.724 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Protactinium
- Category
- actinide
- Appearance
- bright, silvery metallic luster
- Summary
- It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume +4 and even +2 or +3 states.
- Discovered by
- William Crookes
- Melting point
- 1841 K
- Boiling point
- 4300 K
- Density
- 15.37 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Uranium
- Category
- actinide
- Appearance
- None
- Summary
- It is a silvery-white metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.
- Discovered by
- Martin Heinrich Klaproth
- Melting point
- 1405.3 K
- Boiling point
- 4404 K
- Density
- 19.1 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Neptunium
- Category
- actinide
- Appearance
- silvery metallic
- Summary
- A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus.
- Discovered by
- Edwin McMillan
- Melting point
- 9123 K
- Boiling point
- 4447 K
- Density
- 20.45 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Plutonium
- Category
- actinide
- Appearance
- silvery white, tarnishing to dark gray in air
- Summary
- It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states.
- Discovered by
- Glenn T. Seaborg
- Melting point
- 912.5 K
- Boiling point
- 3505 K
- Density
- 19.816 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Americium
- Category
- actinide
- Appearance
- silvery white
- Summary
- This member of the actinide series is located in the periodic table under the lanthanide element europium, and thus by analogy was named after the Americas. Americium was first produced in 1944 by the group of Glenn T.Seaborg from Berkeley, California, at the metallurgical laboratory of University of Chicago.
- Discovered by
- Glenn T. Seaborg
- Melting point
- 1449 K
- Boiling point
- 2880 K
- Density
- 12 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Curium
- Category
- actinide
- Appearance
- silvery metallic, glows purple in the dark
- Summary
- This element of the actinide series was named after Marie and Pierre Curie – both were known for their research on radioactivity. Curium was first intentionally produced and identified in July 1944 by the group of Glenn T. Seaborg at the University of California, Berkeley.
- Discovered by
- Glenn T. Seaborg
- Melting point
- 1613 K
- Boiling point
- 3383 K
- Density
- 13.51 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Berkelium
- Category
- actinide
- Appearance
- silvery
- Summary
- It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the University of California Radiation Laboratory where it was discovered in December 1949.
- Discovered by
- Lawrence Berkeley National Laboratory
- Melting point
- 1259 K
- Boiling point
- 2900 K
- Density
- 14.78 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Californium
- Category
- actinide
- Appearance
- silvery
- Summary
- The element was first made in 1950 at the University of California Radiation Laboratory in Berkeley, by bombarding curium with alpha particles (helium-4 ions). It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all the elements that have been produced in amounts large enough to see with the unaided eye (after einsteinium).
- Discovered by
- Lawrence Berkeley National Laboratory
- Melting point
- 1173 K
- Boiling point
- 1743 K
- Density
- 15.1 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Einsteinium
- Category
- actinide
- Appearance
- silver-colored
- Summary
- It is the seventh transuranic element, and an actinide. Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952, and named after Albert Einstein.
- Discovered by
- Lawrence Berkeley National Laboratory
- Melting point
- 1133 K
- Boiling point
- 1269 K
- Density
- 8.84 g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Fermium
- Category
- actinide
- Appearance
- None
- Summary
- It is a member of the actinide series. It is the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic quantities, although pure fermium metal has not yet been prepared.
- Discovered by
- Lawrence Berkeley National Laboratory
- Melting point
- 1800 K
- Boiling point
- None K
- Density
- None g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Mendelevium
- Category
- actinide
- Appearance
- None
- Summary
- A metallic radioactive transuranic element in the actinide series, it is the first element that currently cannot be produced in macroscopic quantities through neutron bombardment of lighter elements. It is the antepenultimate actinide and the ninth transuranic element.
- Discovered by
- Lawrence Berkeley National Laboratory
- Melting point
- 1100 K
- Boiling point
- None K
- Density
- None g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Nobelium
- Category
- actinide
- Appearance
- None
- Summary
- It is named in honor of Alfred Nobel, the inventor of dynamite and benefactor of science. A radioactive metal, it is the tenth transuranic element and is the penultimate member of the actinide series.
- Discovered by
- Joint Institute for Nuclear Research
- Melting point
- 1100 K
- Boiling point
- None K
- Density
- None g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here
×
Lawrencium
- Category
- actinide
- Appearance
- None
- Summary
- It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radioactive metal, lawrencium is the eleventh transuranic element and is also the final member of the actinide series.
- Discovered by
- Lawrence Berkeley National Laboratory
- Melting point
- 1900 K
- Boiling point
- None K
- Density
- None g/cm3
- Read more at:
- Wikipedia
- Image

- More images and info:
- here









































































